MSD partners with health technology company to help optimise lung cancer care
November 2024
MSD has teamed up with health technology company Axana, and 12 lung cancer clinicians from across Europe to develop a new digital solution to speed up lung cancer care.
The ‘NextGen MDT’ programme aims to help clinicians to make cancer care more efficient and, ultimately, improve patient outcomes.
Lung cancer care has become increasingly complex1, with numerous tests which must be completed before clinicians can diagnose and treat patients,2 and many treatment options available.1 However, these tests can be difficult to coordinate2 and involve different hospital departments or NHS services.3
Clincians specialising in lung cancer gather weekly in multidisciplinary team (MDT) meetings to review each patient’s case and dicuss treatment options.4 If clinicians don’t have all of the information that they need prior to the MDT meeting, treatment decisions can be delayed, which can have a negative impact on patient outcomes.4
At MSD, we’re determined to tackle this problem. We know that, when it comes to cancer, time is of the essence.
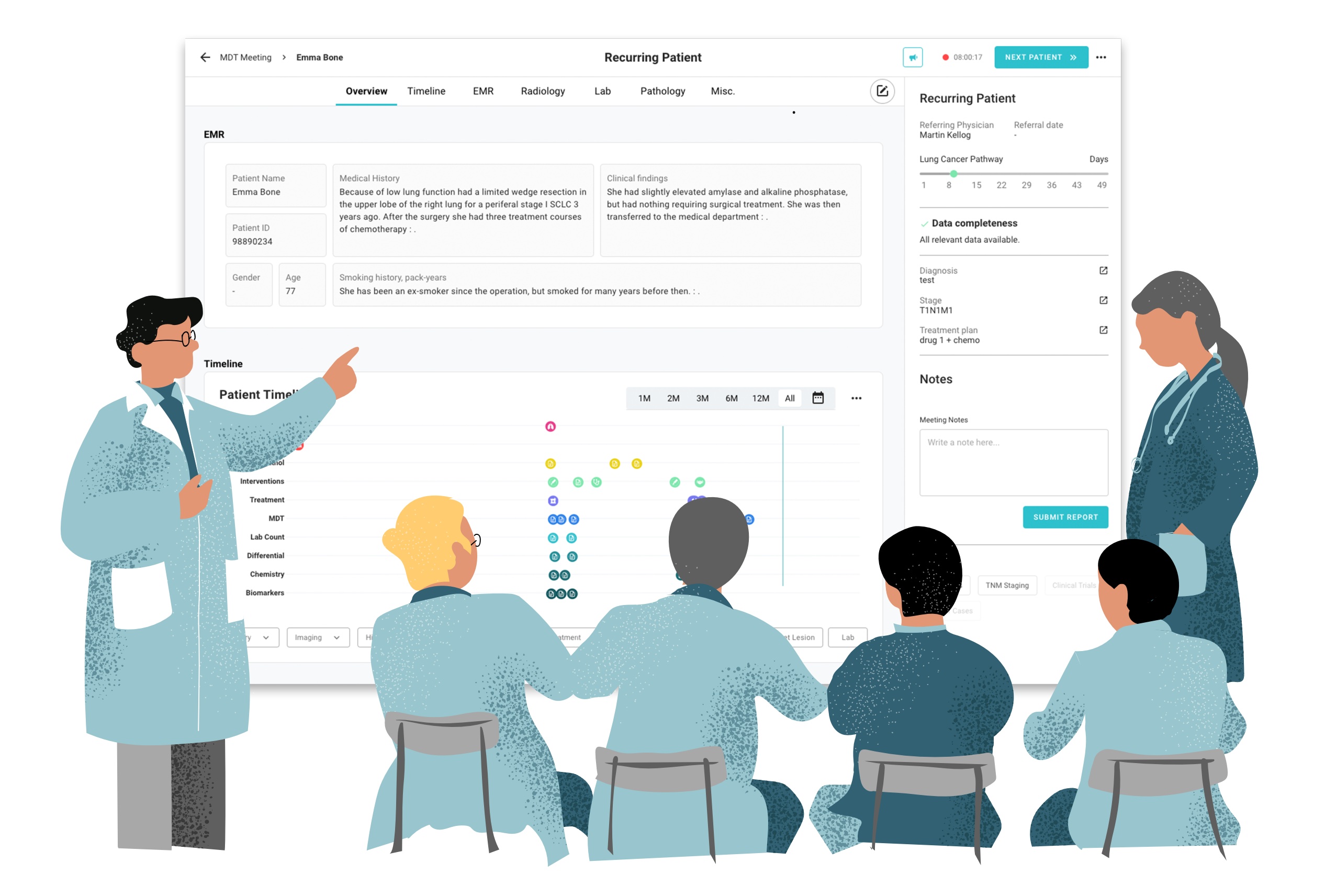
The NextGen MDT programme will strive to give clinicians the time and information that they need to care for their patients. Through the use of intelligent software, the programme aims to integrate patient information into one unified dashboard – simplifying the process of connecting different hospital information systems so that all of the necessary insights about a patient’s cancer are in one place, enabling clinicians to make treatment decisions at the MDT meeting.
Our ultimate goal is to improve the lung cancer patient pathway – reducing the workload of clinicians while speeding up patient access to timely, optimal treatment.
Professor Neal Navani, Respiratory Medicine Consultant and Clinical Lead for Lung Cancer Services at University College London Hospitals NHS Foundation Trust supported the development of the NextGen MDT programme. Commenting on the project, he said:
“This ambitious NextGen MDT project will help to streamline and improve our cancer MDT discussions, allowing us to make better decisions which will benefit our patients and healthcare systems.”
Rachel Houlding, National Oncology Healthcare Director at MSD UK, said:
“Early diagnosis and treatment of lung cancer is crucial to improving patient outcomes.5 Making the patient pathway more efficient is one way to helping patients get the right care for them, sooner – and we believe there is a crucial role that technology can play to achieve this. We are delighted to be working alongside Axana and clinicians across Europe develop such a solution, and look forward to entering the next phase of activity in 2025.”
Jeroen van Duffelen, CEO of Axana, said:
“At Axana, we harness the best minds in data integration and artificial intelligence to address critical challenges in oncology. Our goals are twofold: to enhance clinician efficiency and to expedite patient treatments. Our software streamlines patient data collection and coordination of multidisciplinary team meetings facilitating efficient decision-making by clinical teams. We anticipate that our software will optimize lung cancer care pathways, potentially leading to improved patient outcomes.”
For more information about the NextGen MDT project, please contact corporateaffairsuk@msd.com.
[1] Morabito A, Mercadante E, Muto P, Manzo A, Palumbo G, Sforza V, et al. Improving the quality of patient care in lung cancer: key factors for successful multidisciplinary team working. Explor Target Antitumor Ther. 2024;5:260–77. https://doi.org/10.37349/etat.2024.00217
[2] UK Lung Cancer Coalition (November 2023). Scottish Pathways Matter. Available from: https://www.uklcc.org.uk/sites/default/files/2023-10/Final%20UKLCC%20Scottish%20Pathways%20Matter%20report_Nov%202023.pdf
[3] NHS England. Diagnosis: Lung cancer. Available from: https://www.nhs.uk/conditions/lung-cancer/diagnosis/ [Accessed: September 2024]
[4] Cancer Research UK. Meeting Patient’s Needs: Improving the effectiveness of Multidisciplinary Team meetings in cancer services. 2020. Available from: https://www.cancerresearchuk.org/sites/default/files/full_report_meeting_patients_needs_improving_the_effectiveness_of_multidisciplinary_team_meetings_.pdf
[5] Cancer Research UK. Why is early diagnosis important? 2023. Available from: https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/why-is-early-diagnosis-important/1000#:~:text=Around%206%20in%2010%20people,at%20the%20most%20advanced%20stage.
GB-NON-10247 | November 2024